By Samille Abada, | March 22, 2017
FDA spokeswoman Andrea Fischer said that the agency is now working on specific guidelines to make it clear how human cells, tissues and products should be regulated. (YouTube)
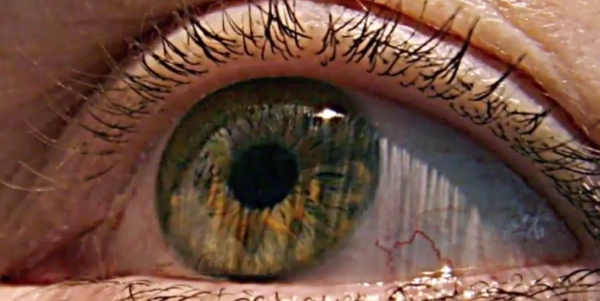
Stem cells have been increasingly popular as it promises treatment for various disorders. But recently, three patients reportedly went blind after stem-cell treatment at a Florida clinic.
The Sunrise stem cell clinic offers the procedure for a wide range of chronic disorders and diseases, but it is largely unregulated. The Sunrise facility has no medical facility license for stem cell operations, according to Florida's Agency for Health Care Administration. Its doctors and nurses are not even regulated by the Florida Department of Health. Both of these agencies claim that the U.S. Food and Drug Administration has the authority to impose regulations on stem cell operations, according to Sun Sentinel.
Like Us on Facebook
However, the guidelines are not yet clear when it comes to adipose stem cells. FDA spokeswoman Andrea Fischer said that the agency is now working on specific guidelines to make it clear how human cells, tissues, and products should be regulated. She claims that the agency has been giving warnings for years to let the public know whether they are part of the FDA-regulated clinical study or not.
Dr. Thomas Albini, an associate professor of clinical ophthalmology at the University of Miami's Bascom Palmer Eye Institute, said that the standards to be followed by these clinics are not clear. "If someone were licensed, that license would be on the line," he said.
According to The New York Times, the clinic has not been sanctioned after the three women went blind after following the procedures in 2015. The women paid $5,000 each for the treatment. Two of these women sued for failure to warn, negligence, and allegations about the product. But the case was subsequently dismissed as they both settled.
-
Use of Coronavirus Pandemic Drones Raises Privacy Concerns: Drones Spread Fear, Local Officials Say

-
Coronavirus Hampers The Delivery Of Lockheed Martin F-35 Stealth Fighters For 2020

-
Instagram Speeds Up Plans to Add Account Memorialization Feature Due to COVID-19 Deaths

-
NASA: Perseverance Plans to Bring 'Mars Rock' to Earth in 2031

-
600 Dead And 3,000 In The Hospital as Iranians Believed Drinking High-Concentrations of Alcohol Can Cure The Coronavirus

-
600 Dead And 3,000 In The Hospital as Iranians Believed Drinking High-Concentrations of Alcohol Can Cure The Coronavirus

-
COVID-19: Doctors, Nurses Use Virtual Reality to Learn New Skills in Treating Coronavirus Patients