By Jacques Strauss, | May 15, 2017

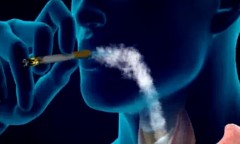
One of the hardest things for some to do is to quit smoking. (YouTube)
A promising cancer treatment drug is being developed by an Anglo-Swedish multinational pharmaceutical and biopharmaceutical company. The new immunotherapy drug promises a greater chance of survival for lung cancer patients.
AstraZeneca Pharmaceutical is currently testing a drug that could halt the progression of stage 3 cancer. The new immunotherapy drug is known as durvalumab and may also be called by its brand name, Imfinzi
Like Us on Facebook
The drug has been approved in the United States. However, this approval is intended for patients suffering from advanced bladder cancer and not for lung cancer. Astra has, therefore, been talking to regulating agencies to acquire a license for the drug for lung cancer treatment, according to Sky.
The drug works by assisting the body's immune cells to fight and kill cancer cells. To assess the efficacy of the treatment for lung cancer, 26 trials are taking place across 26 countries.
The aim of the treatment drug is to alleviate or help lung cancer patients who require treatment beyond the usual chemotherapy. AstraZeneca's chief medical officer Sean Bohen has said that the company is looking "forward to working with regulatory authorities around the world to bring Imfinzi to lung cancer patients as soon as possible."
As of the current writing, immunotherapy is not the primary course for treating lung cancer. Surgery remains the top option along with radiation therapy, chemotherapy, and targeted therapy.
However, more patients and experts are turning to a much-improved immunotherapy. There are fewer side-effects when one undergoes immunotherapy as the treatment is designed mainly to boost the body's natural defense against diseases such as cancer.
Check out the best ways of avoiding lung cancer in the video below:
-
Use of Coronavirus Pandemic Drones Raises Privacy Concerns: Drones Spread Fear, Local Officials Say

-
Coronavirus Hampers The Delivery Of Lockheed Martin F-35 Stealth Fighters For 2020

-
Instagram Speeds Up Plans to Add Account Memorialization Feature Due to COVID-19 Deaths

-
NASA: Perseverance Plans to Bring 'Mars Rock' to Earth in 2031

-
600 Dead And 3,000 In The Hospital as Iranians Believed Drinking High-Concentrations of Alcohol Can Cure The Coronavirus

-
600 Dead And 3,000 In The Hospital as Iranians Believed Drinking High-Concentrations of Alcohol Can Cure The Coronavirus

-
COVID-19: Doctors, Nurses Use Virtual Reality to Learn New Skills in Treating Coronavirus Patients