By Koofi Saa, | September 04, 2016
FDA Bans Antibacterial Soaps After Manufacturers Could not Prove its Efficacy and Safety
The U.S. Food and Drug Administration (FDA) has banned the sale of soaps marketed as antiseptics after manufacturers could not prove the efficacy of the active ingredients in these products.
The FDA banned the sale of antibacterial soaps on Friday, saying producers could not provide data to prove its long-term safety for users. The soap industry could not also prove that antibacterial soaps are better than normal soap and water, the New York Times reported.
Like Us on Facebook
"Consumers may think antibacterial washes are more effective at preventing the spread of germs but we have no scientific evidence that they are any better than plain soap and water," said Dr. Janet Woodcook, FDA's director for Drug Evaluation and Research. "In fact, some data suggests that antibacterial ingredients may do more harm than good over the long-term."
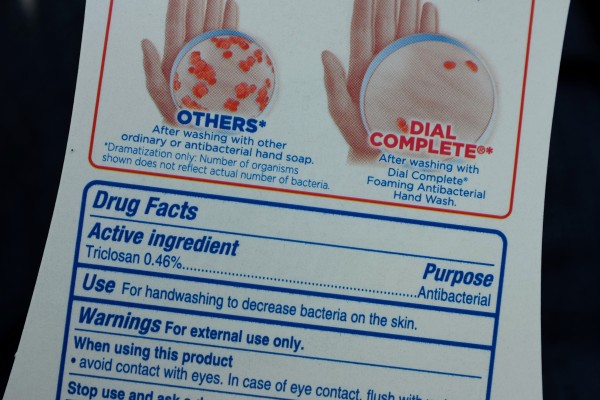
Authorities have given the soap industry a year to stop using the active chemicals in antibacterial soaps. These chemicals, which are about 19 (including triclosan, and triclocarban), are used in the production of most liquid and bar soaps.
"Companies will no longer be able to market antibacterial washes with these ingredients because manufacturers did not demonstrate that the ingredients are both safe for long-term daily use and more effective than plain soap and water in preventing illness and the spread of certain infections," the FDA report read.
The soap industry has been given another year to prove that lesser-known chemicals used in soap production (such as benzalkonium chloride, benzethonium chloride, and chloroxylenol) are effective and safe.
Although authorities have not banned the use of antibacterial hand sanitizers, the FDA has announced that it is currently investigating the active ingredients in these products. The FDA has, however, recommended that people wash their hands with soap and water or use hand sanitizers with more than 60 percent alcohol.
-
Use of Coronavirus Pandemic Drones Raises Privacy Concerns: Drones Spread Fear, Local Officials Say

-
Coronavirus Hampers The Delivery Of Lockheed Martin F-35 Stealth Fighters For 2020

-
Instagram Speeds Up Plans to Add Account Memorialization Feature Due to COVID-19 Deaths

-
NASA: Perseverance Plans to Bring 'Mars Rock' to Earth in 2031

-
600 Dead And 3,000 In The Hospital as Iranians Believed Drinking High-Concentrations of Alcohol Can Cure The Coronavirus

-
600 Dead And 3,000 In The Hospital as Iranians Believed Drinking High-Concentrations of Alcohol Can Cure The Coronavirus

-
COVID-19: Doctors, Nurses Use Virtual Reality to Learn New Skills in Treating Coronavirus Patients