By Arthur Dominic J. Villasanta , | March 30, 2017
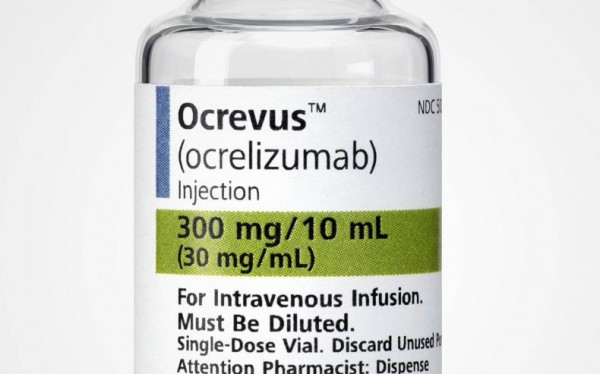
Ocrevus.
The U.S. Food and Drug Administration (FDA) on March 28 approved Ocrevus (ocrelizumab) to treat adult patients with relapsing forms of multiple sclerosis (MS) and primary progressive multiple sclerosis (PPMS).
This is the first drug approved by the FDA for PPMS. Ocrevus is an intravenous infusion developed as a treatment for multiple sclerosis by Genentech, Inc., a subsidiary of Swiss multinational healthcare company Hoffmann-La Roche.
Like Us on Facebook
MS is a chronic, inflammatory, autoimmune disease of the central nervous system that disrupts communication between the brain and other parts of the body.
It is among the most common causes of neurological disability in young adults and occurs more frequently in women than men.
For most people with MS, episodes of worsening function (relapses) are initially followed by recovery periods (remissions). Over time, recovery may be incomplete, leading to progressive decline in function and increased disability. Most people experience their first symptoms of MS between the ages of 20 and 40.
PPMS is characterized by steadily worsening function from the onset of symptoms, often without early relapses or remissions. The U.S. Centers for Disease Control and Prevention (CDC) estimates that 15 percent of patients with MS have PPMS.
The efficacy of Ocrevus for the treatment of relapsing forms of MS was shown in two clinical trials in 1,656 participants treated for 96 weeks. Both studies compared Ocrevus to another MS drug, Rebif (interferon beta-1a). In both studies, the patients receiving Ocrevus had reduced relapse rates and reduced worsening of disability compared to Rebif.
In a study of PPMS in 732 participants treated for at least 120 weeks, those receiving Ocrevus showed a longer time to the worsening of disability compared to placebo.
"Multiple sclerosis can have a profound impact on a person's life," said Billy Dunn, M.D., director of the Division of Neurology Products in the FDA's Center for Drug Evaluation and Research.
"This therapy not only provides another treatment option for those with relapsing MS, but for the first time provides an approved therapy for those with primary progressive MS."
-
Use of Coronavirus Pandemic Drones Raises Privacy Concerns: Drones Spread Fear, Local Officials Say

-
Coronavirus Hampers The Delivery Of Lockheed Martin F-35 Stealth Fighters For 2020

-
Instagram Speeds Up Plans to Add Account Memorialization Feature Due to COVID-19 Deaths

-
NASA: Perseverance Plans to Bring 'Mars Rock' to Earth in 2031

-
600 Dead And 3,000 In The Hospital as Iranians Believed Drinking High-Concentrations of Alcohol Can Cure The Coronavirus

-
600 Dead And 3,000 In The Hospital as Iranians Believed Drinking High-Concentrations of Alcohol Can Cure The Coronavirus

-
COVID-19: Doctors, Nurses Use Virtual Reality to Learn New Skills in Treating Coronavirus Patients